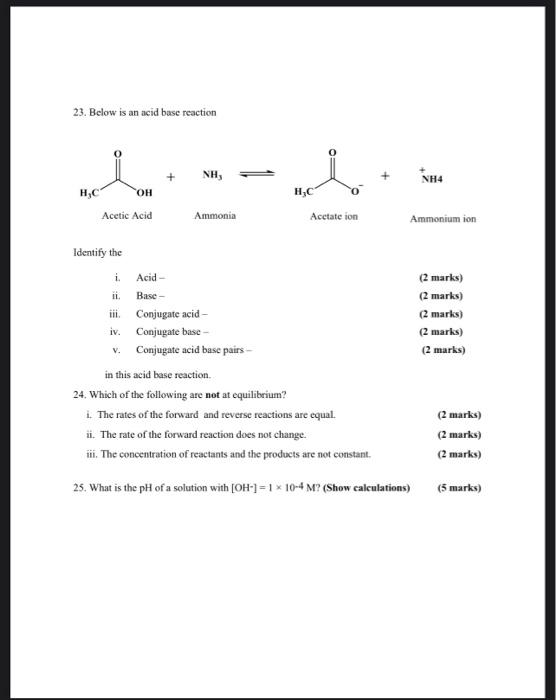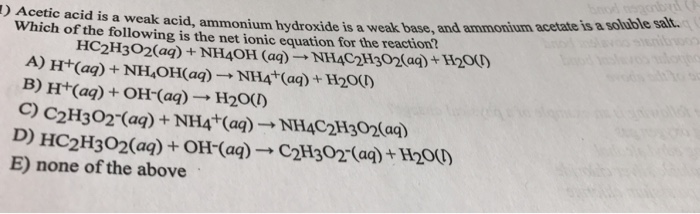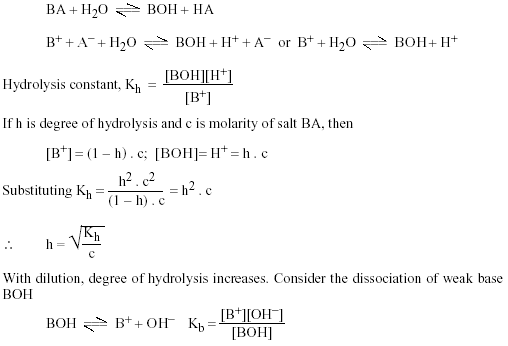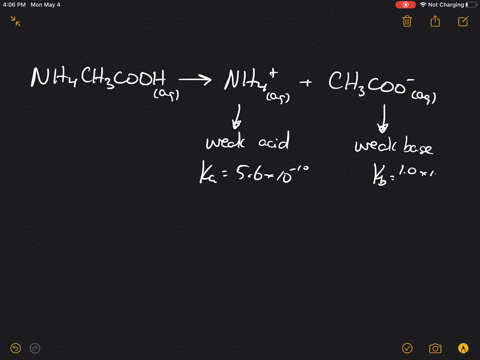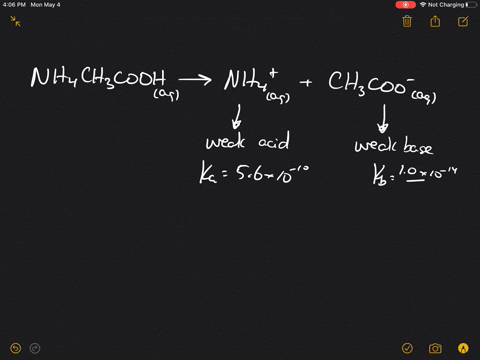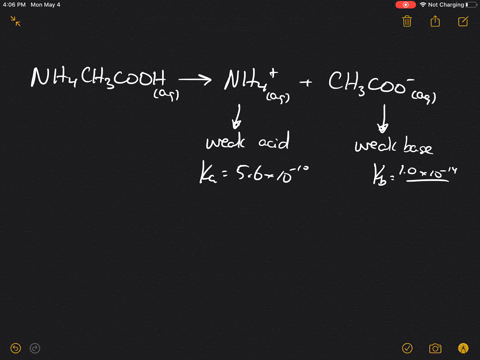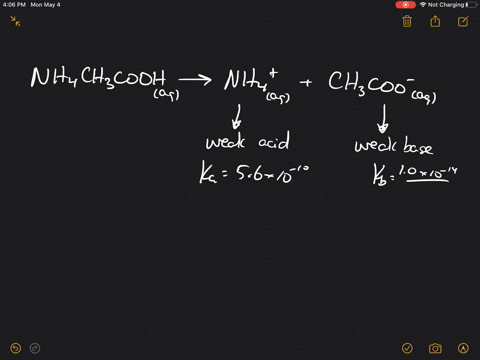
SOLVED:Which of the following salts produces an acidic solution in water: ammonium acetate, ammonium nitrate, or sodium formate?

The pK(a) of acetic acid and pK(b) of ammonium hydroxide are 4.76 and 4.75 respectively. Calculate the pH of ammonium acetate solution.
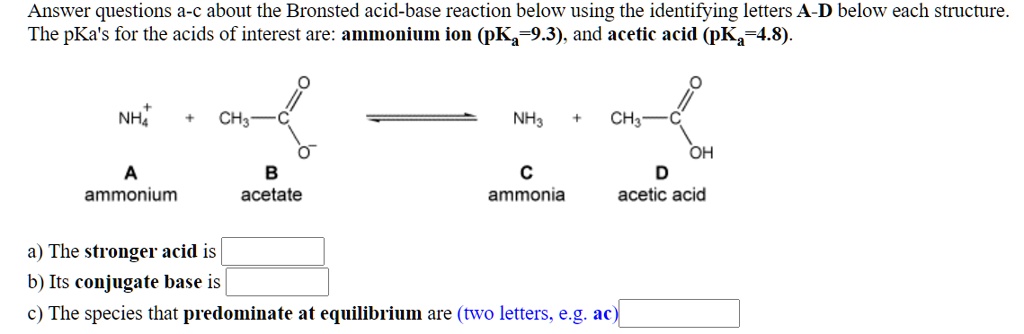
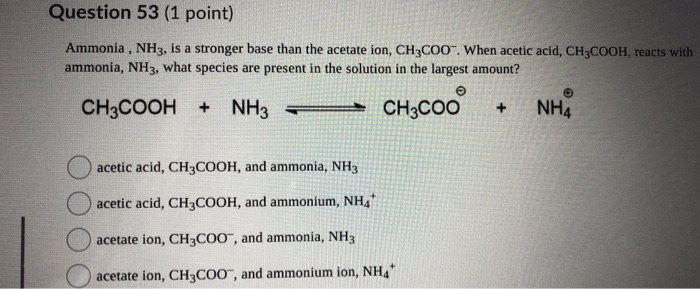
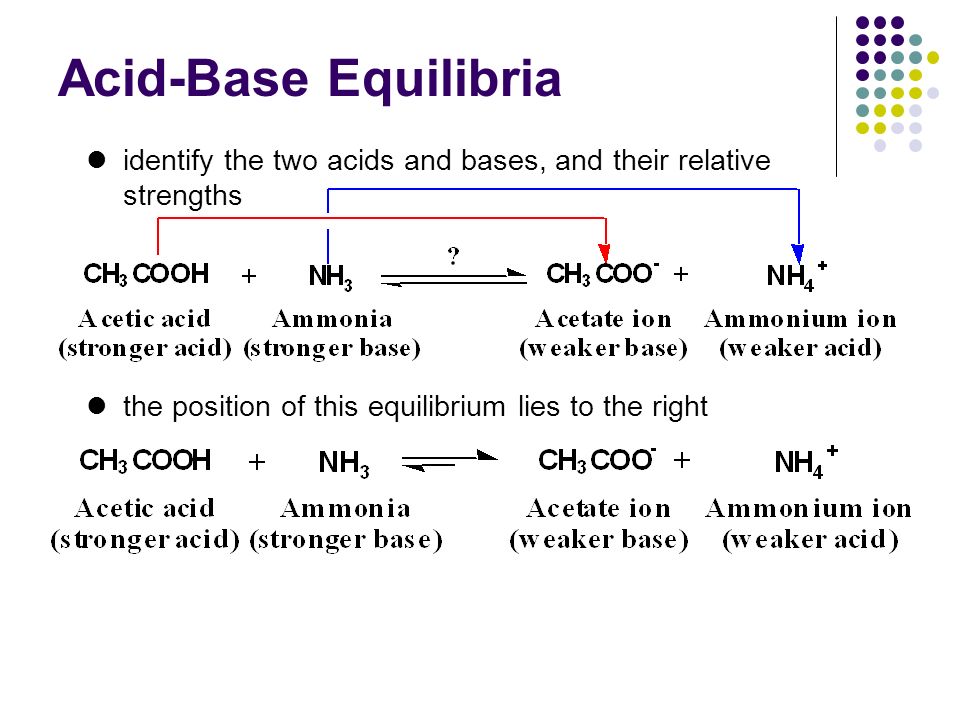
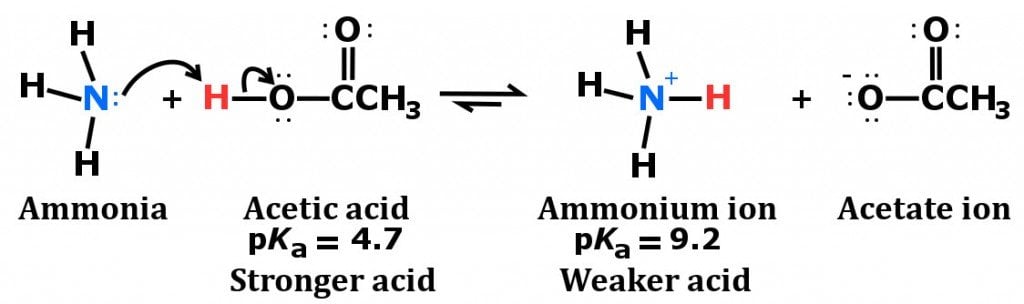
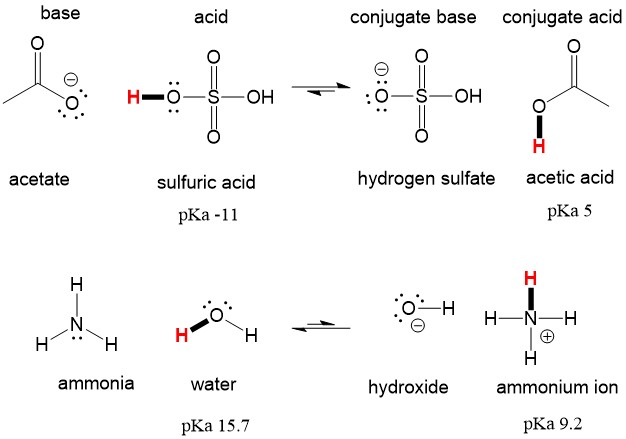
SOLVED: Answer questions a-c about the Bronsted acid-base reaction below uSing the identifying letters A-D below each structure. The pKa's for the acids of interest are: ammonium ion (pKa-9.3). and acetic acid (

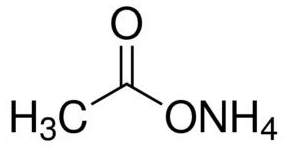
Ammonium acetate (C<sub>2</sub>H<sub>7</sub>NO<sub>2</sub>) - Structure, properties , Production, Uses and FAQs of Ammonium acetate.
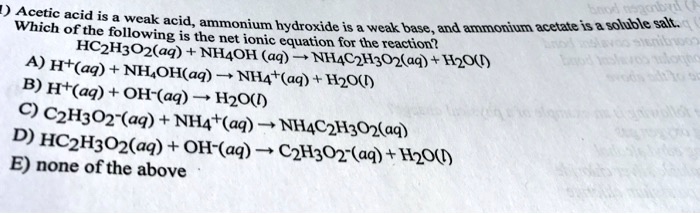
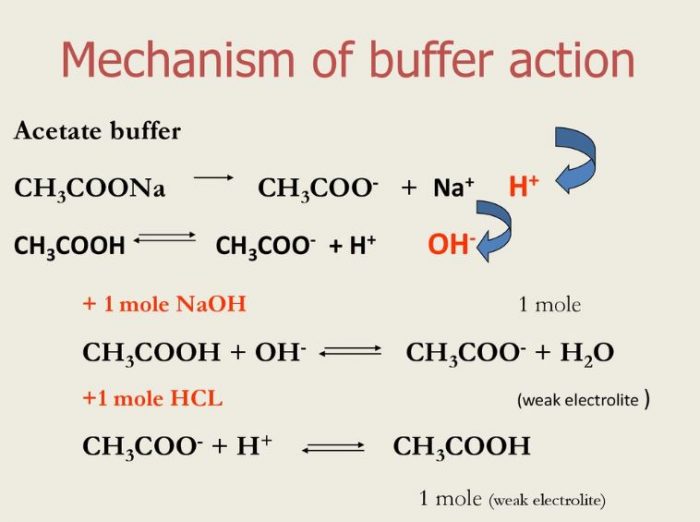
SOLVED: ) Acetic acid is Which of the wcak following acid, ammonium hydroxide is weak base , and ammonium acctate is soluble salt HC2H3Oz(aq) is the net ionic equation for the reaction?

Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram
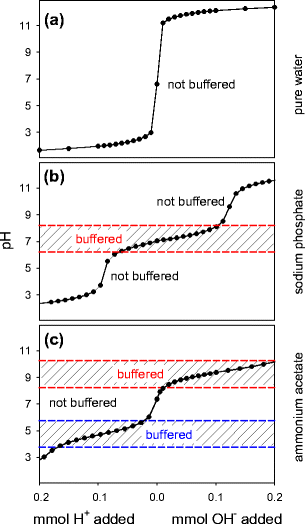
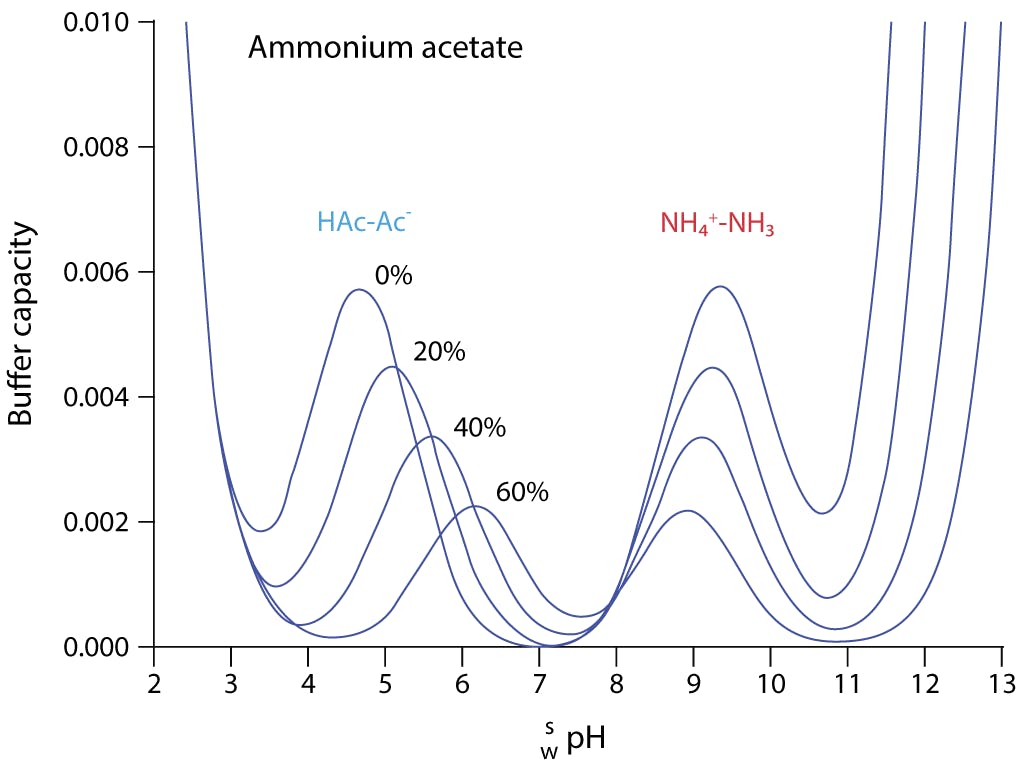
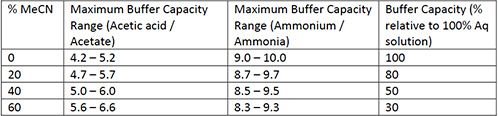
Addressing a Common Misconception: Ammonium Acetate as Neutral pH “Buffer” for Native Electrospray Mass Spectrometry | SpringerLink